Table Of ContentCOORDINATIONOFMRNA3'ENDFORMATIONANDNUCLEAREXPORT
BYANUCLEARPOLY(A)-BINDINGPROTEIN
By
KEITHROBERTNYKAMP
ADISSERTATIONPRESENTEDTOTHEGRADUATESCHOOL
OFTHEUNIVERSITYOFFLORIDAINPARTIALFULFILLMENT
OFTHEREQUIREMENTSFORTHEDEGREEOF
DOCTOROFPHILOSOPHY
UNIVERSITYOFFLORIDA
2003
Dedicatedtomygrandparents,parentsandbrothers,
mywife,Dawn,andson,Jonas.
ACKNOWLEDGEMENTS
Iwouldliketothankmymentor,MauriceSwanson,forprovidingmewith
the resources and intellectual support necessary to complete my doctoral
dissertation. Duringmygraduatestudies,hehaschallengedmetodefendmy
hypotheseswithunequivocaldataandhasequippedmewiththetoolsneeded
foralifetimeofscientificinquiry. Ialsothankmycommitteemembers,Alfred
Lewin, Stephen Sugrue, and Thomas Yang, fortheirvaluable assistance at
importanttimesthroughoutmygraduatecareer. I extend aspecialthanksto
JamesDahlberg,fromtheUniversityofWisconsin-Madison,fortakingtimeoutof
hishecticscheduletoserveasmyoutsideexaminerinGainesville. Importantly,I
thank past and present members ofthe Swanson Lab, in particular James
Anderson, Ron Hector, and Carl Urbinati, for their help with many of the
experiments described in this manuscript. I would also like to thank Lionel
Minvielle-Sebastia forproviding the in vitro polyadenylation results and John
Aitchisonforidentifyingpurifiedproteinsbymassspectrometry. Finally,mywife,
Dawn,deservesspecialmentionbecausewithoutherloveandsupportnoneof
thiswouldhavebeenpossible.
ni
TABLEOFCONTENTS
ACKNOWLEDGEMENTS
iii
ABSTRACT vii
INTRODUCTION 1
IntegratingRNAPIITranscriptionandNuclearProcessingEvents 6
SynthesisofPre-mRNA 6
TranscriptionElongationandGenomeMaintenance 9
RNAPIIRegulatesPre-mRNACapping 10
Pre-mRNASplicingandRecruitmentoftheSpliceosome 12
RegulationofAlternativeSplicingbySRProteinsandhnRNPs 15
ATP-dependentRemodelingoftheSpliceosomeduringSplicing 17
TerminalExonDefinitionRequirestheCapand3'CleavageSite 19
RNAPIIandtheSpliceosomeConnectSplicingtoTranscription 21
3'EndCleavageandPolyadenylation 23
Monitoring3'EndIntegrityduringTranscriptionElongation 27
CoordinatingNuclearProcessingEventsandmRNAExport 29
GeneralMechanismsofNucear/CytoplasmicTransport 29
mRNAExportRequiresaComplexArrayofFactors 31
Pre-mRNASplicingFactorsAreRequiredformRNAExport 33
TREXLinksTranscription,SplicingandmRNAExport 37
ConnectionsbetweenPolyadenylationandmRNAExport 38
MATERIALSANDMETHODS 43
YeastandBacterialCultureMedia 43
YeastStrainsandPlasmids 44
NucleicAcidIsolationProcedures 47
CellTransformation 49
IV
YeastGeneticManipulations 50
YeastTotalCellProteinIsolation 50
FluorescenceInSituHybridizationandCellularImmunofluorescence 51
InVitro3'EndProcessingAssays 52
FilterBindingAssays 53
TandemAffinityPurification 53
PreparationofPolyclonalAntiseraandMonoclonalAntibodies 55
Poly(A)TailLengthDetermination 56
InVitroGSTPull-downExperiments 56
PhosphataseTreatmentwithXPhosphatase 58
RESULTS 59
ResearchObjectives 59
Nab2pBindsPoly(A)RNAandLimitsPoly(A)TailLengthInVitro 61
ExpressionandPurificationofRecombinantGST-Nab2p-His6Protein 62
rNab2pBindswithHighAffinitytoPoly(A)RNAHomopolymers 62
Nab2pRestrictsPoly(A)TailLengthInVitro 65
NAB2LimitsPoly(A)TailLengthsandPromotesmRNAExportInVivo 72
NuclearTargetingofPab1pSuppressesthenab2AGrowthDefect 73
Nab2pIsRequiredForPoly(A)TailLengthControlandmRNAExport.... 76
Nab2pAssociateswithFactorsRequiredformRNAExport 85
PurificationofNab2pT-associatedProteins 85
Nab2pandKap104pFormanAbundantComplexinYeastExtracts 89
Nab2pCo-purifieswithNuclearmRNA-bindingProteins 95
mRNAExportIsInhibitedbyaMutationintheNESofNab2p 100
Mex67pInteractswiththeN-terminusofNab2p 101
nab2-20StabilizesMex67p-Nab2pInteractionsInVitro 104
Nab2pIsPhosphorylatedinStrainsDefectiveformRNAExport 110
DISCUSSION 115
Nab2pIsRequiredforPoly(A)TailLengthRestriction 115
PotentialRoleforNab2pinPreventingNucleolarRetentionofmRNA 121
Nab2pInteractswithImportandExportReceptors 126
Limitations 130
Conclusions 132
APPENDIX 133
REFERENCES 138
BIOGRAPHICALSKETCH 167
VI
AbstractofDissertationPresentedtotheGraduateSchool
OftheUniversityofFloridainPartialFulfillmentofthe
RequirementsfortheDegreeofDoctorofPhilosophy
COORDINATIONOFMRNA3'ENDFORMATIONANDNUCLEAREXPORTBY
ANUCLEARPOLY(A)-BINDINGPROTEIN
By
KeithRobertNykamp
December2003
Chair:MauriceS.Swanson
Major:MolecularGeneticsandMicrobiology
EukaryoticmessengerRNAistranscribed inthenucleusandtranslated
intoproteininthecytoplasm. Priortoexportfromthenucleus,mRNAsmustbe
cappedatthe5'endwitha7-methylguanylate(m7G),intronsmustberemoved
viasplicing,andapolyadenylatetailmustbeaddedtothe3'end. Splicingand
polyadenylation are carried out by large macromolecular machines and are
coordinatelyregulated by RNApolymerase II (RNAP II)transcription. During
transcription, membersofa largefamilyofnuclearRNA-binding proteins,the
heterogeneousnuclearRNAbindingproteins(hnRNPs),bindtonascentRNAPII
transcripts. Co-transcriptionalassociationofhnRNPswithnascentpre-mRNAis
thoughtto regulatethe specificityand timing ofsubsequent RNAprocessing
events by influencing the recruitment of the spliceosome and
cleavage/polyadenylationfactors. Followingpre-mRNAprocessing,exportfrom
VII
the nucleus is promoted by interactions between the mRNAand specialized
nuclearexportfactors.
AlthoughhnRNPsassociatewithnuclearpoly(A)+RNAandarerequired
forefficient mRNA export in vivo, their role in mRNA export has remained
controversial. SuggestionshavebeenmadethathnRNPsbindnon-specifically
topre-mRNAanddonotdirectlyrecruitmRNAexportreceptors. Alternatively,
hnRNPsmayactattheinterfacebetweenmRNAprocessingeventsandnuclear
export, orchestrating the temporal recruitment ofmRNA exportfactors after
processing has occurred in vivo. Thegoal ofthe research presented inthis
reportwas to testthe latter hypothesis using the yeast hnRNP Nab2p. My
resultsdemonstratethatNab2pisanuclearpoly(A)-bindingprotein,requiredfor
bothterminationofpolyadenylationand mRNAexport. Surprisingly,thesetwo
processescan beuncoupled innab2mutantstrains,andNab2pinteractswith
thenuclearmRNAexportfactorMex67p. Basedontheseresults,theproposition
ismadethatNab2pcoordinatestheterminationofpolyadenylationwithmRNA
exportinvivo.
V111
INTRODUCTION
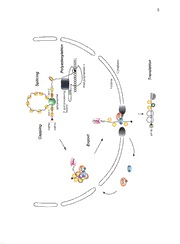
ThesynthesisofmessengerRNA(mRNA)inaeukaryoticcelloccursin
the nucleus and involves transcription of pre-mRNA by RNA polymerase II
(RNAPII)followedbyseveralprocessingsteps,includingcapping,splicing,and
polyadenylation(seeFigure1). ThetranslationofmRNAintoproteins,however,
occursinthecytoplasm. Importantly,nuclearporecomplexes(NPCs)allowfor
the translocation of RNAs and proteins through the nuclear envelope (NE)
(reviewedinRyanandWente,2000).
Compartmentalizationoftranscription and pre-mRNAprocessing inthe
nucleus and protein translation in the cytoplasm allows for much greater
regulation ofgene expression than could be attained otherwise (reviewed in
HoodandSilver, 1999;KomeiliandO'Shea,2000). Forexample,p53normally
shuttlesbetweenthenucleusandcytoplasm,butlocalizestothenucleusduring
stressconditionswhereitincreasesthetranscriptionofstressresponsegenes
(Middeleretal.,1997). Interestingly,theinabilityofp53tolocalizeinthenucleus
correlateswiththeproliferationofseveraltumorsemphasizingtheimportanceof
proteinimportregulation(Molletal.,1995;Shlampetal.,1997).
Theadditionofa7-methylguanylatecaptothe5'endandapolyadenylate
tailtothe3'endofmRNAprovidesadditionalopportunitiesfortheeukaryoticcell
to regulate gene expression (Shatkin and Manley, 2000). Under normal
conditions, the cytoplasmic cap-binding protein (elF4E) and poly(A) binding
protein(PABP),protectagainstdegradationandaidinproteinsynthesisthrough
interactionswiththecapandpoly(A)tail,respectively(reviewedinWiluszetal.,
2001). AU-richelement(ARE)RNA-bindingproteins,suchasAUF1/hnRNPD
and tristetraprolin (TTP) bypass PABP-dependent mRNA stabilization by
promotingmRNAdecaywhenboundto3'untranslated(UTR)regionsofproto-
oncogeneandcytokinemRNAs(Laietal.,1999;Laroiaetal.,1999;Loflinetal.,
1999). Alternatively, HuRantagonizesthebindingofAUF1/hnRNPDtoAREs
andaugmentsPABP-dependentstabilizationofthesemRNAs(FanandSteitz,
1998;Gallouzietal.,2000). OtherARE-bindingproteins(TIARandTIA-1)have
beendemonstratedtoinhibitPABP-dependentre-initiationofproteinsynthesis
(Gueydan etal., 1999; Piecyk et al., 2000). Importantly, ARE mutations or
aberrant levels ofARE-binding proteins correlatewith a varietyofdiseases,
includingautoimmunity,arthritis,myeloidhyperplasia,andtumorigenesis(Gouble
etal.,2002; Kontoyiannisetal., 1999;Tayloretal., 1996). Properlyregulated
mRNAturnoverisclearlyveryimportantforcellviability.
Alternativepre-mRNAsplicing alsogives risetoextraordinarylevelsof
gene regulation by increasing the number of different proteins that can be
producedbyasinglegene(SmithandValcarcel,2000). TheD.melanogaster
Down'sSyndromecelladhesion molecule(Dscam)geneprovidesanextreme
exampleofthisphenomenon(Schmuckeretal.,2000). Theauthorspredictthat
-38,000 distinct protein isoforms are generated by alternative pre-mRNA
splicing. Given so much diversity and complexity, it is not surprising that