Table Of ContentZootaxa 4407 (3): 361–375 ISSN 1175-5326 (print edition)
Article ZOOTAXA
http://www.mapress.com/j/zt/
Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition)
https://doi.org/10.11646/zootaxa.4407.3.4
http://zoobank.org/urn:lsid:zoobank.org:pub:F95D4394-8A44-4331-9D2D-ED8ABBE8AF17
Cactodera chenopodiae (Nematoda: Heteroderidae), a new species of
cyst nematode parasitizing common lambsquarter
(Chenopodium album) in Liaoning, China
YAXING FENG1, DONG WANG1, DONGXUE XIAO1, TIAGO JOSÉ PEREIRA2, YUANHU XUAN1,
YUANYUAN WANG1, XIAOYU LIU1, LIJIE CHEN1, YUXI DUAN1 & XIAOFENG ZHU1,3
1College of Plant Protection, Nematology Institute of Northern China, Shenyang Agricultural University, Shenyang, China 110866
2Department of Nematology, University of California, Riverside, 900 University Avenue, Riverside, CA 92521, USA
3Corresponding author. E-mail: [email protected]
Abstract
A new species of cyst nematode, Cactodera chenopodiae n. sp., parasitizing common lambsquarter, Chenopodium album
L., is described from native vegetation in Liaoning, China. Cactodera chenopodiae n. sp. has a circumfenestrate pattern
typical of the genus and is morphologically similar to C. cacti Krall & Krall, 1978. However, in the new species, females
and cysts show a larger L/W ratio whereas second-stage juveniles (J2s) have a longer hyaline region. The new species is
also morphologically similar to C. milleri Graney & Bird, 1990, but the J2s differ by a larger b ratio and longer tail. Based
on DNA sequences of the 28S and ITS rRNA, C. chenopodiae n. sp. comes close to C. estonica Krall & Krall, 1978, al-
though it is distinct from the latter with respect to the presence of a punctate eggshell and larger b ratio in the J2s. Although
morphometric comparisons with additional Cactodera species show the overlapping of diagnostic morphological charac-
ters, our phylogenetic analyses based on both rRNA genes support C. chenopodiae n. sp. as a unique lineage.
Key words: genus Cactodera, ribosomal genes, morphology, phylogeny, plant-parasitic nematode, taxonomy
Introduction
During the summer of 2015, second-stage juveniles (J2s) of a cyst-forming nematode were detected from soil
around common lambsquarter, Chenopodium album L., a plant widely distributed in China. In the US, Graney &
Bird (1990) also reported cyst nematodes (i.e., Cactodera milleri Graney & Bird, 1990) parasitizing weeds
including C. album, C. amaranticolor Coste & Reyn, and C. quinoa Willd. Since 2015, additional soil sampling
has been performed in China revealing the presence of cysts and a few white females attached to roots of the plant
host (i.e., C. album). A detailed study of these nematodes indicated a small vulval cone and circumfenestrate
pattern typical of Cactodera Krall & Krall, 1978. Morphological and molecular analyses of the material were
performed and compared with the 14-other valid Cactodera species (Subbotin et al., 2010; Cid Del Prado Vera &
Subbotin, 2014), thus revealing the nematode to be a new species. Herein, we describe this cyst nematode as
Cactodera chenopodiae n. sp. using morphological and molecular characters.
Material and methods
Nematode isolation. Females, cysts and J2s of C. chenopodiae n. sp. were collected from the natural habitat of the
host plant, C. album, in Beiling Park, Shenyang, Liaoning Province, China (41°50’38” N, 123°25’44” E, 51 m
a.s.l.). Although soil samples were collected seven times (monthly from May to October, 2015), males were not
found. Cysts, J2s–J4s and females were extracted from soil samples using standard centrifugal-flotation and
Fenwick methods (Fenwick, 1940). Immature stages and females were dissected directly from the roots under a
stereomicroscope (Nikon SMZ800) for further morphological characterization.
Accepted by N. Kanzaki: 7 Mar. 2018; published: 11 Apr. 2018 361
Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0
Morphological study. Nematodes in water were killed, fixed and dehydrate according to Cid Del Prado Vera
& Subbotin (2012). Nematodes were then processed to glycerin using a modified Seinhorst (1959) method as
described by Cid Del Prado Vera & Subbotin (2012). The specimens were hand-picked from the dish and then
mounted on glass slides using a paraffin wax ring method (de Maeseneer & d’Herde, 1963). Permanent slides they
were then examined under light microscopy (LM) for morphological characterization. Measurements and drawings
were made using a drawing tube mounted on an Olympus BX53 compound microscope. For scanning electron
microscopy (SEM), specimens were post fixed in an aqueous solution of 4% osmium tetroxide for 12 hours and
then dehydrated in an series of ethanol solutions (20–100%) for 20 min at each concentration (Cid Del Prado Vera
et al., 2012). The specimens were critical point-dried and coated with gold-palladium and then observed under a
field emission SEM (Zeiss Ultra Plus) at 5 kV.
Nematode-infected plant tissues were treated for observation using a modified acid-fuchsin staining-destaining
procedure (Bybd Jr et al., 1983). Washed roots were placed in a 150 ml beaker with 50 ml tap water and 20 ml of
chlorine bleach (5.25 % NaClO) resulting in a solution of 1.5% NaClO. After occasional agitation during 4 min in
the NaClO, the roots were rinsed in flowing water (30–45 sec) and then allowed to soak in tap water for another15
min to remove residual NaClO. The material was then drained and transferred to a beaker containing 30 ml of
water to which was added 1 ml of stain (3.5 g acid fuchsin, 250 ml acetic acid, and 750 ml distilled water). This
solution was then heated to boiling for about 30 sec. After cooling to room temperature, excess stain was removed
by rinsing in flowing water. The root material was then placed in 20–30 ml of glycerin acidified with 2–3 drops of
5N HCl, heated to boiling, and cooled. For microscopic examination the roots were pressed between glass plates or
microscope slides.
Molecular characterization. DNA was extracted from 5 specimens (single cyst containing J2s and eggs)
using a Worm lysis buffer [Subbotin et al., 2010, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl, 0.045%
2
Tween 20, and 0.045% Nonidet P 40] in conjunction with Proteinase K (20 mg/ml, Takara Bio Inc.). Three
ribosomal RNA (rRNA) genes (18S, 28S and ITS) and one mitochondrial DNA fragment (COI) were partially
amplified via PCR. Detailed primer sequences are summarized in Supplement TABLE 1. Detailed protocols for the
molecular procedures (i.e., DNA extraction, and PCR conditions) used in this study are as described in Subbotin et
al. (2010). All PCR reactions included negative controls.
Cloning and sequencing. Initial DNA sequences of C. chenopodiae n. sp. obtained through direct sequencing,
showed some ambiguous sites in upstream and downstream of the sequence, positive PCR products were cloned
and re-sequenced. Briefly, DNA was excised from 1.2% TAE buffered agarose gels using the Tiangen Gel
Extraction Kit (Tiangen Biotech Co., Ltd.), cloned into the pMD-T vector (Takara Bio Inc.) and transformed into
Top10 Competent Cells. For each specimen, 5 clones were isolated using the blue/white selection and submitted to
PCR with vector primers. One clone of each specimen was sequenced using the universal primer of pMD-T vector
(Takara Bio Inc.). No intraspecific/genomic variation was observed.
Phylogenetic analyses. New DNA sequences obtained in the present study were submitted to the GenBank
database under the accession numbers: ITS (KY475583), 28S (KY475584), 18S (MG566084) and COI
(MG744314). Since there were no COI sequences and only 3 18S sequences available in NCBI database. Only ITS
and 28S sequences phylogenetic tree were built. The sequences were separately aligned using MEGA 7 (http://
www.megasoftware.net) with default parameters. Additional previously published DNA sequences of Cactodera
species and representatives of other circumfenestrate cyst nematodes were also included in the alignment for
phylogenetic context (Bernard et al., 2010; Cid Del Prado Vera et al., 2014; Duan et al., 2012; Ferris et al., 1999,
2004; Maafi et al., 2003; Qin et al., 2004; Sabo et al., 2010; Subbotin et al., 2001, 2006, 2011; Uehara et al., 2005).
Outgroup taxa for phylogenetic analyses were chosen according to previous studies of cyst nematodes (Subbotin et
al., 2006, 2011).
Phylogenetic relationships among sequences were estimated with maximum likelihood (ML), maximum
parsimony (MP), and Bayesian inference (BI). MP analyses were performed in MEGA 7 using heuristic searches
and SPR branch swapping to seek for the most parsimonious trees (max. tree number = 100). Gaps in the alignment
were treated as missing data. Nonparametric bootstrap analysis (BS), using 1000 pseudo replicates, was used to
assess branch support. For ML and BI analyses, MrModelTest 2 (Nylander, 2004.) was used to determine the
model of DNA evolution that best fit the data. The model inferred base on the Akaike Information Criterion (AIC)
values were GTR + I + G for ITS and GTR + G for 28S. ML analyses were performed in MEGA 7
(Bootstrap=1000). BI analyses were run on MrBayes 3.1.2 (Huelsenbeck & Ronquist, 2001) with a random seed,
362 · Zootaxa 4407 (3) © 2018 Magnolia Press FENG ET AL.
and four Metropolis-coupled Markov chain Monte Carlo (MCMC) for 2,000,000 generations with a subsampling
frequency of 100. The log-likelihood values stabilized after approximately 5,000 generations; sample points
obtained prior to convergence were discarded as burn-in. BI trees were edited using FigTree 1.3.1 (Rambaut, 2009)
and Adobe Illustrator® CS4 (Adobe Systems).
Results
Cactodera chenopodiae* n. sp.
(Figs. 1–5, Tables 1)
*specific epithet after the host, Chenopodium album L.,
Measurements. See Table 1.
Description. Females. Body ovate to rounded or subspherical in shape with small vulval cone (Fig. 1C, 1D),
female body pearly-white (Fig. 1G, 1H). Females with small vulval cones having slightly protruding lips (Fig. 1C,
1D). Female full of eggs and gelatinous egg sac not observed (Fig. 1I). Outer cuticular layer marked by a rugose
pattern. Head slightly set off from the elongate and protruding neck, stylet and stylet knobs well developed (Fig.
1A). Excretory pore located at same level as end of isthmus (Fig. 1B). Anus distinct (Fig.1E, 1F).
Cysts. Rounded to lemon-shaped, from light to dark brown with small vulval cone (Figs. 1K, 1L, 4A, 5F). Cyst
surface with zigzag pattern at mid-body and not prominent on surface of the vulval cone (Fig. 4C, 4D). Cone is
circumfenestrate and lacks an underbridge, bullae and vulval denticles (Fig. 1C, 1D, 4D). Anus distinct and
encircled within a disc-like cuticular region (Fig. 4B).
Males. Not found.
J2s. Vermiform, tapering anteriorly and posteriorly (Fig. 2A). Stylet knobs rounded to slightly projecting
anteriorly (Fig. 2B). Lip region slightly set off with four annuli. In en face view, an elongated labial disc
surrounded by four submedial and two lateral lips (Fig. 4E, 5G). Excretory pore near level of gland lobe,
hemizonid was one and a half annulus above excretory pore (Fig. 2B, 2E, 2F 5B). Lateral field with four lines with
outer two ridges partially areolated along the body. (Figs. 2G, 4F, 5C). Tail tapering, with hyaline region; the
hyaline region is often shorter than the stylet. Transition to hyaline region usually clearly demarcated by an outline
that is V-shaped, U-shaped, or rarely sloping ventrally (Fig. 2C, 2D, 5D).
Eggs. Surface with heavy punctations visible both under LM and SEM (Figs. 1J, 4G–I). Only found inside
cysts.
Type host and locality. Common lambsquarter, C. album in the Beiling Park, Shenyang, Liaoning province,
China. Coordinates: 41°50’38” N, 123°25’44” E, 51 m a.s.l.
Type material. Holotype female, seven paratype females, twenty paratype cysts and twenty paratype J2s were
deposited for curation in the collection of the Nematology Institute of Northern China (NINC), Shenyang
Agricultural University, Shenyang, China.
Biology. Cactodera chenopodiae n. sp. was found on roots of C. album among other native vegetation of
China. On the type host, the cyst nematode ranges from endoparasitic to semi-endoparasitic. Juveniles were
detected on the roots of the host plant by acid fuchsin staining(Fig.3A). Moreover, some maturing juveniles
(presumed to be sedentary J2s, J3s and J4s) were found with the anterior portion of their body penetrating into the
roots (Fig. 3B–C).
Diagnosis and relationships. Cactodera chenopodiae n. sp. belongs to the genus Cactodera which includes
species characterized by a small vulval cone and a circumfenestrate terminal pattern. The new species can be
differentiated from the fourteen other Cactodera species by a combination of morphological and molecular
characters.
The ranges of many morphological characters of C. chenopodiae overlap with those of other Cactodera
species and isolates, including with certain C. cacti isolates from different regions of the US [e.g., Michigan,
Graney & Bird (1990)]; yet, morphometric analyses of C. chenopodiae n. sp. demonstrate that the means for these
morphological features are distinctive relative to C. cacti. For example, females of C. chenopodiae n. sp. have a
larger L/W ratio [1.6 (1.4–1.7) vs. 1.2 (1.0–1.4)], and J2s have a smaller b ratio [3.8 (3.6–4.1) vs. 6 (5.6–6.8)] as
well as a longer hyaline region [22.7 μm (17.5–28.4) vs. 17.6 μm (13.7–20.5)]. Cactodera chenopodiae n. sp. is
CACTODERA CHENOPODIAE N. SP. FROM CHENOPODIUM ALBUM Zootaxa 4407 (3) © 2018 Magnolia Press · 363
also distinguished from C. milleri, known to parasitize common lambsquarter in Michigan, US (Graney & Bird,
1990), by the cyst having an anus set off within a distinctive disc-shaped cuticular pattern (Fig. 1E–F, 4B) and by
having a greater fenestral diameter [23.5 μm (19.9–26.3) vs. 18.7 μm (14.3–22.0)]. Moreover, J2s stages have a
larger b ratio [3.8 (3.6–4.1) vs. 2.9 (2.6–3.1)] and a longer hyaline region [22.7 μm (17.5–28.4) vs. 18.2 μm (14.6–
21.2)] when compared to C. milleri.
TABLE 1. Morphometric measurements of Cactodera chenopodiae n. sp.
Paratype
Stage Character Holotype Mean ± SE Range CVa
Female
n 8
Length 566 632.2 ± 31.8 504.1–713.4 23.1
Width 361 393.2 ± 13.6 316.8–566.18 21.4
L/W ratio 1.6 1.61 ± 0.02 1.44–1.72 6.3
Vulval slit 13.55 13.4± 0.55 12.9–16.7 9.5
Vulva to anus distance 52.45 52.6± 0.58 50.1–55.36 3.7
Cyst
n 20
Length 486.1 ± 9.20 423.4–585.4 8.7
Width 333.8 ± 7.65 283.0–398.1 10.5
L/W ratio 1.5 ± 0.03 1.2–1.7 8.6
Fenestral diam 23.5 ± 0.55 19.9–26.3 10.6
J2
n 20
Length 490.3 ± 5.47 438.2–539.3 5
Width 22.3 ± 0.46 19.5–27.6 9.2
Stylet 24.0 ± 0.27 21.9–25.9 5.1
Stylet shaft and knobs 13.4 ± 0.10 8.3–10.0 3.4
Stylet knobs to DGO 3.9 ± 0.12 3.1–4.3 13.6
Head end to excretory pore 112.2± 1.10 103.9–121.3 4.4
Tail 45.7 ± 0.70 39.1–50.6 6.9
Hyaline region 22.7 ± 0.53 17.5–28.4 10.7
a 22.0± 0.42 18.0–24.7 8.6
b 4.1± 0.02 3.46–4.7 2.9
c 10.8 ± 0.17 9.8–12.6 6.9
c′ 3.2 ± 0.08 2.7–4.0 11.4
m 0.4 ± 0.01 0.4–0.5 7
o 0.2 ± 0.01 0.1–0.2 14.4
H (%)(Hyaline region /Tail × 100) 49.0± 0.01 39.2–61.5 10.6
egg
n 25
L 112.9 ± 1.13 107.1–120.9 5
W 46.8 ± 0.53 42.5–52.5 5.6
L/W 2.4 ± 0.03 2.0–2.7 5.7
All measurements are in μm.
a CV: coefficient of variation.
364 · Zootaxa 4407 (3) © 2018 Magnolia Press FENG ET AL.
FIGURE 1. Light micrographs of Cactodera chenopodiae n. sp. (Female and Cyst) A. Stylet of female; B. Neck of female; C–
D. Vulva and anus of female; E–F. Posterior ends of female showing vulval slit; G. Immature females on roots; H. Females; I.
Mature female (full of eggs); J. Egg; K. Cyst; L. Cysts. (Scale bars: A–B, E, F, J =20 μm, C–D = 50 μm, G, I = 200 μm, H= 500
μm, K = 100 μm, L=1mm.)
As for closely related species (i.e. based on molecular data), C. chenopodiae n. sp. is distinguished from C.
estonica (Kirjanova & Krall, 1963) Krall & Krall, 1978, by the eggshell pattern (i.e., punctate in C. chenopodiae
vs. smooth in C. estonica) and larger b ratio in J2s [3.8 (3.6–4.1) vs. 2.8 (2.7–2.9)]. In addition, C. chenopodiae n.
sp. differs from C. rosae Cid del Prado & Miranda, 2008 by a longer hyaline region [22.7 μm (17.5–28.4) vs. 6.3
μm (4.0–6.8)]
Molecular profiles and phylogenetic status
The molecular characterization and position of C. chenopodiae n. sp. within Cactodera was evaluated using two
ribosomal regions (i.e., ITS and 28S). In the phylogenetic tree inferred from the D2–D3 expansion segments of the
28S rRNA gene, all Cactodera species grouped together forming a clade (Fig. 6). Specifically, C. chenopodiae n.
CACTODERA CHENOPODIAE N. SP. FROM CHENOPODIUM ALBUM Zootaxa 4407 (3) © 2018 Magnolia Press · 365
sp. was a sister taxon of C. rosae in subclade A, which also includes C. torreyanae Cid Del Prado & Subbotin,
2014. Yet, C. cacti and C. galinsogae Tovar Soto, Cid Del Prado, Nicol, Evans, Sandoval Islas & Martinez Garza,
2003, were sister to one another in subclade B, and relatively more divergent from species in subclade A (Fig. 6).
In the ITS phylogeny, molecular variation within Cactodera was better represented with the inclusion of DNA
sequences representing four additional Cactodera species in the molecular analyses. All Cactodera species were
monophyletic in the ITS phylogeny, and although intraspecific variation occurs, this seems to be relatively low as
suggested by the short branch lengths (Fig. 7). Indeed, for C. chenopodiae n. sp. intraspecific variation was not
detected as multiple clones representing five different specimens showed identical DNA sequences.
FIGURE 2. Light micrographs of Cactodera chenopodiae n. sp. (J2) A. Entire body of J2; B: Anterior region of J2; C–D: Tail
of J2(U or V); E. Hemizonid of J2; F. Excretory pore of J2; G. Lateral field of J2. (Scale bars: A = 50 μm, B–G = 10 μm)
The phylogenetic tree based on ITS rRNA gene differed slightly from that based on 28S. Four main subclades
can be identified in the ITS phylogeny: subclade A includes the same species reported in the 28S phylogeny
(without C. chenopodiae n. sp.), in addition to C. salina Baldwin, Mundo-Ocampo & McClure, 1997, and C.
weissi (Steiner, 1949) Krall & Krall, 1978; subclade B contains C. estonica and C. chenopodiae n. sp.; subclade C
bears C. galinsogae and C. milleri; and subclade D is only represented by sequences of C. cacti.
Overall, the 28S and ITS phylogenies were congruent with respect to the monophyly of the different genera
366 · Zootaxa 4407 (3) © 2018 Magnolia Press FENG ET AL.
included in the analyses. Within Cactodera, however, species relationships differed slightly between the two
phylogenies [e.g., C. chenopodiae n. sp. as sister to C. rosae (28S rRNA tree) or sister to C. estonica (ITS rRNA
tree)]; this difference can be due to the inclusion of more species in the ITS analysis.
FIGURE 3. Light micrographs of Cactodera chenopodiae n. sp. (J3) A–C: J3 in root after root staining; D: J3 off root picked
after centrifugal-flotation. (Scale bars: A–C = 100 μm, D = 50 μm)
Cactodera species known to parasitize common lambsquarter also include C. milleri (Graney & Bird, 1990).
However, phylogenetic analyses based on the ITS rRNA (Fig. 7) suggest that C. chenopodiae n. sp. is genetically
distant from C. milleri 4.9 % (44 bp difference). In fact, genetic divergence between C. chenopodiae n. sp. and
species in subclade A for the ITS gene was about 4.9–5.9 % (44–53 bp difference); species in subclade B (C.
estonica) was 1.6–1.8 % (16–17 bp difference); species in subclade C was about 4.0–4.9 % (36–44 bp difference);
species in subclade D (C. cacti) was 9.5–9.6 % (86 bp difference). The molecular variation among the different
clades supports C. chenopodiae n. sp. as a unique lineage.
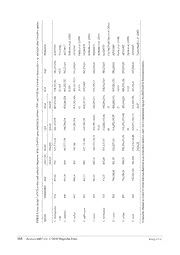
The virtual sequence digestion using Restriction Analysis obtained for C. chenopodiae n. sp. is presented in
Table 2. This RFLP-ITS pattern distinguishes C. chenopodiae n. sp. from comparable profiles available for other
Cactodera species, as shown in Subbotin et al. (2010), and especially from species in subclade A, B and D,
including C. estonica, C. milleri, C. galinsogae, C. rosae, C. torreyanae, C. weissi, C. salina, C. cacti (Table 2). A
size comparison of fragments indicates that the enzyme TaqI is most useful to distinguish the new species from
these eight Cactodera species.
CACTODERA CHENOPODIAE N. SP. FROM CHENOPODIUM ALBUM Zootaxa 4407 (3) © 2018 Magnolia Press · 367
(cid:9)(cid:20)(cid:2)(cid:6)(cid:4)(cid:25)(cid:27)(cid:23)(cid:2)(cid:14)(cid:23)(cid:27)(cid:6)(cid:19)(cid:2)(cid:2)(cid:7)(cid:11)(cid:6)(cid:22)(cid:4)(cid:6)(cid:7)(Cactodera(cid:3) (cid:18)(cid:6)(cid:15)(cid:6)(cid:19)(cid:6)(cid:9)(cid:22)(cid:6)(cid:7)(cid:2) 23/+--$0(cid:2) (cid:29)(cid:27)(cid:4)(cid:7)(cid:2)(cid:7)(cid:23)(cid:21)(cid:20)"((cid:2) 4’+//%+(cid:2) (cid:3)(cid:21)(cid:10)(cid:10)(cid:14)(cid:23)(cid:4)(cid:9)(cid:2)(cid:8)’11%(cid:12)(cid:2)et al. 4%,%11+(cid:2) 4(cid:6)(cid:19)(cid:19)(cid:4)(cid:7)(cid:2)(cid:8)%***(cid:12)(cid:2)et al. 56’,1/%*(cid:2) (cid:3)(cid:21)(cid:10)(cid:10)(cid:14)(cid:23)(cid:4)(cid:9)(cid:2)(cid:8)’1%%(cid:12)(cid:2)et al. 56’,1/%-(cid:2) (cid:3)(cid:21)(cid:10)(cid:10)(cid:14)(cid:23)(cid:4)(cid:9)(cid:2)(cid:8)’1%%(cid:12)(cid:2)et al. 24’%/+--(cid:2) (cid:17)(cid:4)(cid:20)(cid:2)7(cid:6)!(cid:2)(cid:16)(cid:19)(cid:24)(cid:20)(cid:14)(cid:2)8(cid:6)(cid:19)(cid:24)(cid:2)(cid:8)’1%/(cid:12)(cid:2)et al. 4%,%11,(cid:2) 4(cid:6)(cid:19)(cid:19)(cid:4)(cid:7)(cid:2)(cid:8)%***(cid:12)(cid:2)et al. 4%,%11-(cid:2) 4(cid:6)(cid:19)(cid:19)(cid:4)(cid:7)(cid:2)(cid:8)%***(cid:12)(cid:2)et al. 4/*$0*0(cid:2) (cid:29)(cid:24)(cid:9)(cid:27)(cid:24)(cid:2)9(cid:24)(cid:24)(cid:15)(cid:4)(cid:2)(cid:8)’110(cid:12)(cid:2)et al.
(cid:24)
(cid:11)((cid:2)
(cid:7)
’$(cid:2)(cid:15)(cid:14)(cid:19)(cid:2)(cid:2)(cid:9)((cid:2)Cactodera chenopodiae RsaI TaqI -0/.0,-.0/.(cid:2)0$%.’+’.’%,.(cid:2) ’1.%/.*(cid:2),-./’(cid:2) /$-.’0’.%%’.(cid:2)-,’.’+%.,-(cid:2) -%.*.*(cid:2) /1,(0’%(+-(-%--%.’,*.,-(cid:2) (%/(*(*(cid:2) -’*.0//.*(cid:2)-,0.’-/.,-(cid:2) -0,.00-.%/(cid:2)-,,.’-/.,-(cid:2) -0/.0,’.%1.*(cid:2)-$1.’+1.,-(cid:2) /1+.0’,.%’-.(cid:2)--,.’,*.,-(cid:2) %/.*.*(cid:2) /’$.’%*.%’,.(cid:2)--*.’,*.,-(cid:2) %%%.*(cid:2) -/-.0/,.*(cid:2)-,+.’,$.,-(cid:2) (cid:29)9 (cid:29)(cid:17)5=(cid:18)(cid:6)(cid:7)(cid:23)(cid:19)(cid:4)(cid:22)(cid:23)(cid:4)(cid:14)(cid:9)9(cid:24)(cid:11)(cid:11)(cid:6)(cid:19)(cid:12)((cid:3)
& (cid:16)
,(cid:2) $(cid:2) /. %(cid:2) %(cid:2) *. *. ,(cid:2) $. (cid:9)=
(cid:29)#$%(cid:2)(cid:24)(cid:9)(cid:20)(cid:2) MvaI (BstNI) /-*.’+%.’/ /’/.’/,.’’ /%-.’/0.%, ,0(cid:2) /’/.’/+.’% /’*.’/-.’% //’.’/,.%* ’$(cid:2) /’1.’//.%* ’+(cid:2) /’0.’//.’’ 00%.’/*.%* *-.’+(cid:2) (cid:6)((cid:14)(cid:19)(cid:25)=(cid:22)(cid:25)(cid:4)(cid:30)(cid:10)(cid:4)
(cid:7)(cid:2) (cid:26)
(cid:25)(cid:6)(cid:9)(cid:6)(cid:2)(cid:24)(cid:26)(cid:11)!(cid:4)(cid:15)(cid:4)(cid:6)(cid:20)(cid:2)(cid:10)"(cid:2)(cid:11)(cid:19)(cid:4)(cid:26)(cid:6)(cid:19) CfoI (HhaI) 0/$.0’*.’**(cid:2) 0/$.’*/.’-,(cid:2) 0/-.’$,.’-/(cid:2) 0/*(’*/(’0*(cid:2) -(cid:2)0//.%*,.%/-.$%.(cid:2) +,./0(cid:2) 0%’.’,’.%*-.$,.(cid:2) ,1(cid:2) 0//.’*’.%*-.-*(cid:2) /(cid:2)0/’.%*-.%-+.%/1. -*(cid:2) 1.0/0.’-0.%$-.%%’. +(cid:2) (cid:7)((cid:2)(cid:8)(cid:27)(cid:23)(cid:23)(cid:11)<==>>>("(cid:6)(cid:24)(cid:7)(cid:23)(cid:25)(cid:6)(cid:9)(cid:14)
(cid:14)(cid:15)(cid:2)(cid:23)(cid:27)(cid:6)(cid:2)(cid:28)(cid:29)(cid:3)(cid:30)(cid:19)(cid:18)(cid:31) (cid:2) BsuRI (HaeIII) -’-.’++.%+/(cid:2) /$’.’++.%0*(cid:2) +/-.%/1(cid:2) ,0+.%0*.%1,(cid:2) /$$.%*+.%’-.+ -1-.’%0.%*+(cid:2) -1’.’1+.%$%(cid:2) -1’.%*,.%1%.* /-$.’%/.%%$./ ’/.’/.’’(cid:2) (cid:6)(cid:7)(cid:23)(cid:19)(cid:4)(cid:22)(cid:23)(cid:4)(cid:14)(cid:9)(cid:2) (cid:9)(cid:24)!"(cid:7)(cid:4)
(cid:13)(cid:2)(cid:14)(cid:15)(cid:2)(cid:16)(cid:17)(cid:18)(cid:2)(cid:11)(cid:19)(cid:14)(cid:20)(cid:21)(cid:22)(cid:23)(cid:2)(cid:24)(cid:9)(cid:20)(cid:2)(cid:19)(cid:6)(cid:7)(cid:23)(cid:19)(cid:4)(cid:22)(cid:23)(cid:4)(cid:14)(cid:9)(cid:2)(cid:15)(cid:19)(cid:24)(cid:25)(cid:26)(cid:6)(cid:9)(cid:23)(cid:7)(cid:2) )(cid:9)(cid:19)(cid:6)(cid:7)(cid:23)(cid:19)(cid:4)(cid:22)(cid:23)(cid:6)(cid:20)(cid:2)AluI Bsh1236I (BstUI) *+,(cid:2)$*-.$%(cid:2)*+,(cid:2) $*$(cid:2)$0*.0,(cid:2)$*$(cid:2) $$-(cid:2)$/*.0,(cid:2)$$-(cid:2) $$’(cid:2)$,%.’%(cid:2)$$’(cid:2) $$-(cid:2)$,/.’%(cid:2)$/’./0(cid:2) *%-(cid:2)$+$.0+(cid:2)$--.,1(cid:2) $*1(cid:2)+/$.%1,.0,(cid:2)$0%.-*(cid:2) $*0(cid:2)+-%.%1,.0,(cid:2)$0/.-*(cid:2) *11(cid:2)--’.’1,.%/’(cid:2)-*,(01/(cid:2) (cid:6)(cid:7)(cid:21)!(cid:23)(cid:7)(cid:2)(cid:14)(cid:15)(cid:2):(cid:4)(cid:19)(cid:23)(cid:21)(cid:24)!(cid:2)(cid:7)(cid:6);(cid:21)(cid:6)(cid:9)(cid:22)(cid:6)(cid:2)(cid:20)(cid:4)(cid:25)(cid:6)(cid:7)(cid:23)(cid:4)(cid:14)(cid:9)(cid:2)(cid:21)(cid:7)(cid:4)(cid:9)(cid:25)(cid:2)(cid:18)
(cid:11)(cid:12)(cid:2) (cid:9)(cid:2)(cid:19)
(cid:9)(cid:2)(cid:10) (cid:6)(cid:20)(cid:2)(cid:4)
(cid:3)(cid:4)(cid:5)(cid:6)(cid:7)(cid:8)(cid:9)(cid:2)(cid:3)(cid:4)(cid:5)(cid:6)(cid:7)(cid:2)(cid:8)(cid:4) (cid:3)(cid:11)(cid:6)(cid:22)(cid:4)(cid:6)(cid:7)(cid:2) C. chenopodiae (cid:9)((cid:7)(cid:11)( C. estonica C. milleri C. galinsogae C. rosae C. torreyanae C. weissi C. salina C. cacti 4(cid:19)(cid:24)(cid:25)(cid:26)(cid:6)(cid:9)(cid:23)(cid:7)(cid:2)(cid:14)(cid:10)(cid:23)(cid:24)(cid:4)(cid:9)
(cid:3) (cid:2) (cid:13)
368 · Zootaxa 4407 (3) © 2018 Magnolia Press FENG ET AL.
FIGURE 4. SEM micrographs of Cactodera chenopodiae n. sp. A. Cyst; B. Vulval cone with anus; C. Cuticle surface showing
wavy pattern; D. Circumfenestra of cyst; E. Anterior region of J2; F. Lateral field of second-stage juvenile (J2) showing
incomplete annulation; G. Egg; H–I. Pattern on surface of egg;
CACTODERA CHENOPODIAE N. SP. FROM CHENOPODIUM ALBUM Zootaxa 4407 (3) © 2018 Magnolia Press · 369
FIGURE 5. Drawing of Cactodera chenopodiae n. sp. A: Entire body of J2; B: Anterior region of J2; C–D: Tail of J2; E:
Terminal view of cone; F: Cyst; G: Face view of J2 as observed with SEM.
370 · Zootaxa 4407 (3) © 2018 Magnolia Press FENG ET AL.